Tissue-Specific Hydrogels Ameliorate Hepatic Ischemia/Reperfusion Injury in Rats by Regulating Macrophage Polarization via TLR4/NF-κB Signaling
Injectable acellular matrix hydrogels are proven to be potential translational materials to facilitate the repairment in various tissues. However, their potential to repair hepatic ischemia/reperfusion injury (IRI) has not been explored. In this work, they made hepatic acellular matrix (HAM) hydrogels based on the decellularized process and evaluated the biocompatibility and hepatoprotective effects in a rat IRI model. HAM hydrogels supported viability, proliferation, and attachment of hepatocytes in vitro. Treatment with HAM hydrogels significantly attenuated hepatic damage caused by IRI, as evidenced by hepatic biochemistry, histology, and inflammatory responses. Importantly, HAM hydrogels inhibited macrophage M1 (CD68/CCR7) differentiation but promoted M2 (CD68/CD206) differentiation. Additionally, TLR4/NF-κB signaling was found to be involved in the hepatoprotective effect of HAM hydrogels. Collectively, their study reveals that HAM hydrogels ameliorate hepatic IRI by facilitating M2 polarization via TLR4/NF-κB signaling.
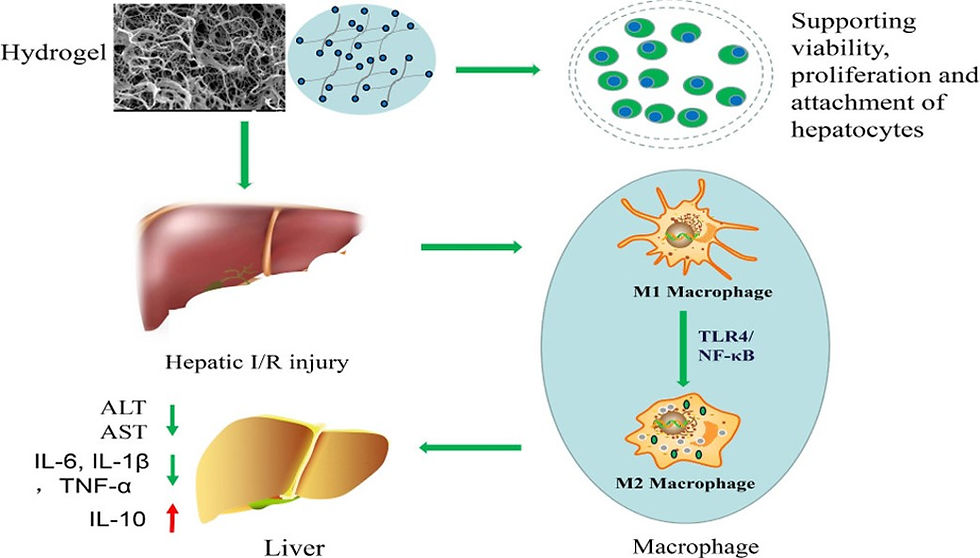
Hepatic ischemia/reperfusion injury (IRI) is widely considered as an important issue influencing the outcomes of various surgeries, such as liver transplantation, trauma, and liver resection. Hepatic IRI can cause severe tissue damage and hepatic necrosis or even graft failure after liver transplantation, resulting in increased mortality. Because hepatic IRI involves multiple complex mechanisms, there is no clinically available therapeutic strategy to prevent this disease thus far. Therefore, developing effective protective strategies to treat hepatic IRI is of paramount importance.
In the past few years, cell therapy based on stem cells has been investigated in preventing hepatic IRI in animal models. Despite the advancements that have been made in treating hepatic IRI, the therapeutic efficacy now is limited due to the poor cell survival in the adverse microenvironment of an injured liver, for example, inflammation, ischemia, and oxidative stress. It has been proposed that injectable hydrogels such as Matrigel, self-assembling peptide hydrogel, and collagen could deliver cells as the carriers, which not only can replace and repair the injured extracellular matrix (ECM) but also regulate the hostile microenvironment of damaged tissue. These injectable hydrogels tried to mimic the hepatic ECM and to offer the appropriate chemical and biological cues to improve cell retention, survival, and enhance the efficacy of cell therapy. However, these hydrogels failed to mimic the complex structural and chemical cues of the natural liver ECM. Considering that providing cells with the appropriate environmental cues is the key element in achieving success in tissue engineering strategy, it thus remains necessary to develop a more suitable hydrogel for liver regeneration.

(A) H&E and immunofluorescence staining of native and acellular hepatic tissues. (B) DNA contents of native and acellular hepatic tissues. (C) Contents of GAG proteins in native and acellular hepatic tissues. Data are means ± s.e.m. ***p < 0.001. All experiments were repeated 3 times. Scale bars are 100 μm. n = 3.
Recently, biologic scaffolds derived from decellularized tissues have emerged as superior scaffolds for a variety of tissue engineering applications. Compared with artificial biomaterials, acellular ECM scaffolds possess better biocompatibility and provide the most appropriate chemical and biological cues that mimic the native microenvironment. Importantly, acellular ECM scaffolds have been solubilized to produce injectable hydrogels. Such ECM hydrogels have several desirable advantages for therapeutic applications, including minimally invasive delivery, ability to quickly fill irregularly shaped spaces, and biologic activity of the native matrix. Thus, more recent efforts have been dedicated to explore the potential clinical application of injectable ECM hydrogels derived from cardiac tissue, skeletal muscle, and the peripheral nervous system.In this regard, several groups have reported that hepatic acellular matrix (HAM) hydrogels can be used to coat tissue culture plates. Other groups also utilized HAM hydrogels to evaluate its effect on hepatocyte function. However, the contributions of HAM hydrogels on hepatic ischemia/reperfusion injury (IRI) have not been explored1.

(A) At room temperature, the solubilized HAM was liquid. (B) At 37 °C, the HAM self-assembled into a hydrogel. (C) Representative SEM images of HAM hydrogels with a nanofiber structure. All experiments were repeated 3 times. Scale bars are 1 μm. n = 3.

(A,B) Live/Dead assay showed the cell viability of BRL-3A cells within HAM hydrogels and gelatin and control groups at days 1 and 3. (C) Quantitative analysis showed that no statistically significant difference was observed in cell viability among the three groups on days 1 and 3. (D) AlamarBlue Viability Assay showed no apparent change in the relative number of live cells among the three groups after culture for 1 and 3 days. Data are showed as means ± s.e.m. All experiments were repeated 3 times. Scale bars are 40 μm. n = 3.

(A,B) Fluorescence staining of F-actin showed that BRL-3A cells within HAM hydrogels spread wider, and their actin filaments were thicker on HAM hydrogels than on gelatin. (C) Quantitative analysis of cell adhesion indicated that no statistically significant difference was observed in the number of adhesive cells between the two groups on days 1 and 3. (D) Adherent area of an individual BRL-3A cell was higher on HAM hydrogels compared to that on gel. Data are showed as means ± s.e.m. *p < 0.05. All experiments were repeated 3 times. Scale bars are 40 μm. n = 3.

(A) 1 h after post-injection, the HAM hydrogels (green) were seen within the injection area in liver tissues of the IRI rats under a fluorescence microscope. (B,C) Dramatic decrease in the serum levels of both AST and ALT was observed in the HAM group when compared with those in the IRI + saline group. (D,E) H&E staining exhibited a marked cell death and tissue damage in the IRI + saline group, which was dramatically reduced in the rats treated with HAM hydrogels. Black arrows indicate the colonization of the HAM hydrogel. Scale bars are 50 μm. n = 5.

(A–C) Dramatic decrease of pro-inflammatory factors IL-6, IL-1β, and TNF-a was observed in the HAM group compared with those in the IRI group. (D) Slight increase of anti-inflammatory IL-10 was observed in the HAM group. All experiments were performed in triplicate. *p < 0.05, ***p < 0.001. n = 5.
In the present study, they first aimed to fabricate an injectable HAM hydrogel based on the decellularized hepatic tissues and to assess its influence on cell behavior (growth, proliferation, and adhesion) in vitro. Furthermore, they evaluated the potential role of the HAM hydrogel in hepatic IRI in the rat model and explored the underlying mechanisms behind its effectiveness.
1. Shuai Li, Chengxiao Liang, Wen Jiang, Jie Deng, Rui Gu, Wei Li, Fuzhou Tian, Lijun Tang, and Hongyu Sun
ACS Biomaterials Science & Engineering 2021 7 (4), 1552-1563
DOI: 10.1021/acsbiomaterials.0c01610



Comments