Gene activated scaffolds incorporating star-shaped polypeptide-pDNA nanomedicines accelerate BTE
- kübra:)

- Jun 12, 2021
- 6 min read
Gene activated scaffolds incorporating star-shaped polypeptide-pDNA nanomedicines accelerate bone tissue regeneration in vivo
Increasingly, tissue engineering strategies such as the use of biomaterial scaffolds augmented with specific biological cues are being investigated to accelerate the regenerative process. For example, significant clinical challenges still exist inefficiently healing large bone defects which are above a critical size. Herein, they describe a cell-free, biocompatible and bioresorbable scaffold incorporating a novel star-polypeptide biomaterial as a gene vector.
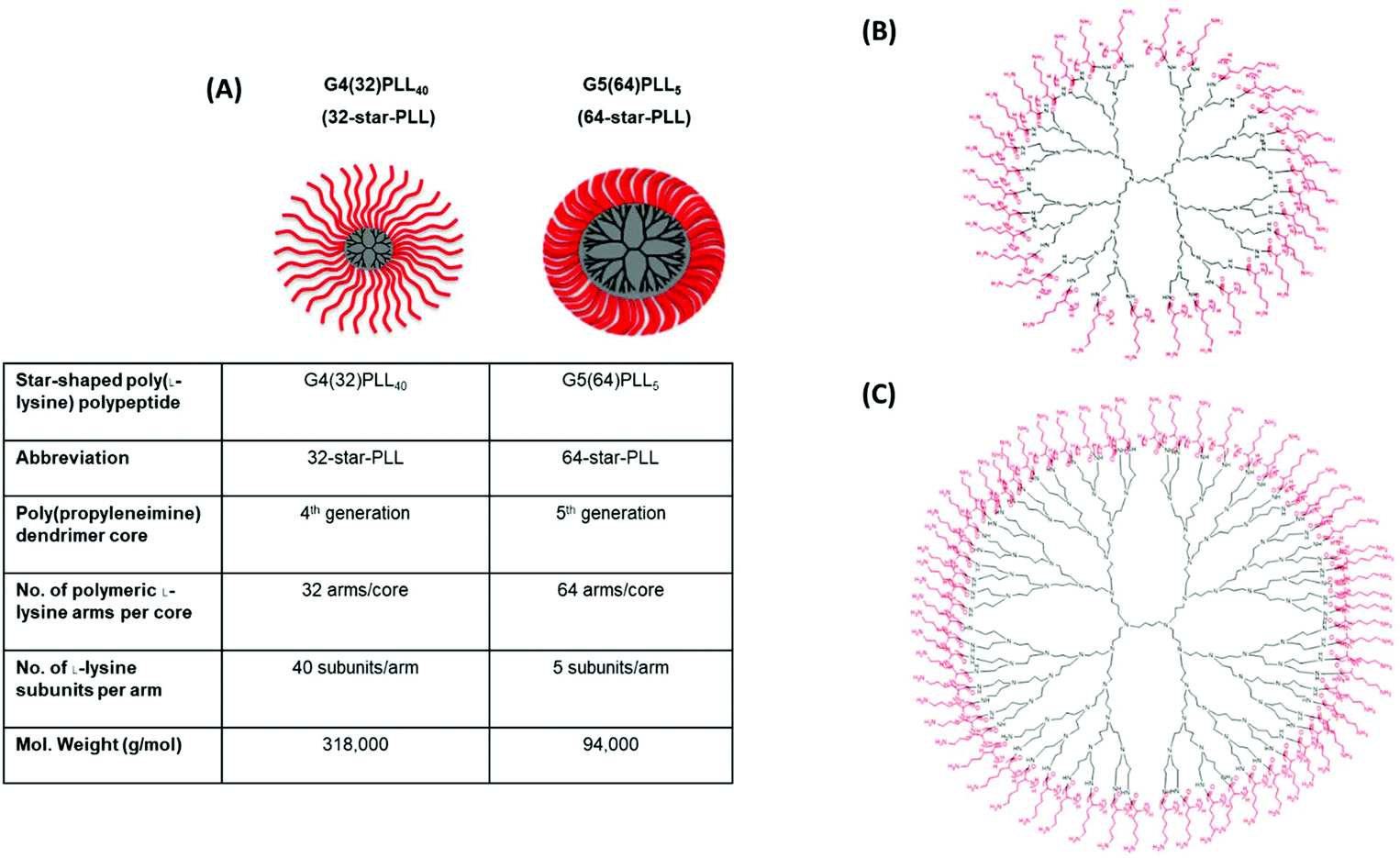
Illustrated above is (A) a structural outline of the two star-PLLs used in this study, namely G4(32)PLL40 (32-star-PLL) and G5(64)PLL5 (64-star-PLL), (B) chemical structure of 32-star-PLL and (C) chemical structure of 64-star-PLL.
This gene-loaded scaffold can accelerate bone tissue repair in vivo in comparison to a scaffold alone at just four weeks post-implantation in a critical-sized bone defect. This is achieved via the in situ transfection of autologous host cells which migrate into the implanted collagen-based scaffold via gene-loaded, star-shaped poly(L-lysine) polypeptides (star-PLLs). In vitro, they demonstrate that star-PLL nanomaterials designed with 64 short poly(L-lysine) arms can be used to functionalize a range of collagen-based scaffolds with a dual therapeutic cargo (pDual) of the bone-morphogenetic protein-2 plasmid (pBMP-2) and vascular endothelial growth factor plasmid (pVEGF).

A dual therapeutic cargo of pBMP-2 and pVEGF delivered using 64-star-PLL facilitates enhanced in vitro MSC mediated osteogenesis within a collagen-CS scaffold. (A) Calcium deposition was assessed following seeding of MSCs onto a collagen-CS scaffold with either 64-star-PLL-pBMP-2 (N/P 5, 5 μg pBMP-2), 64-star-PLL-pVEGF (N/P 5, 5 μg pVEGF) or 64-star-PLL-pDual (N/P 5, 5 μg pDual). The 64-star-PLL-pDual gene activated scaffold exhibited the highest level of calcium deposition 28 days post seeding with MSCs (n = 3). (B) DNA quantification revealed that a significant reduction in DNA levels per scaffold did not occur for any group (n = 3). (C) Representative alizarin red stains from sections of each scaffold group with positive calcium deposition observed as a red deposit. Staining was evident for all groups except the scaffold incubated in (i) GM. GM = scaffold alone in growth medium, OM = scaffold alone in osteogenic medium.
The versatility of this polymeric vector is highlighted in its ability to transfect Mesenchymal Stem Cells (MSCs) with both osteogenic and angiogenic transgenes in a 3D environment from a range of scaffolds with various macromolecular compositions. In vivo, they demonstrate that a bone-mimetic, collagen-hydroxyapatite scaffold functionalized with star-PLLs containing either 32- or 64- poly(L-lysine) arms can be used to successfully deliver this pDual cargo to autologous host cells. At the very early timepoint of just 4 weeks, they demonstrate the 64-star-PLL-pDual functionalised scaffold as a particularly efficient platform to accelerate bone tissue regeneration, with a 6-fold increase in new bone formation compared to a scaffold alone. Overall, this article describes for the first time the incorporation of novel star-polypeptide biomaterials carrying two therapeutic genes into a cell free scaffold which supports accelerated bone tissue formation in vivo.

Star-PLL-pDual collagen-HA scaffolds accelerate bone tissue regeneration in vivo. Four weeks post implantation of gene activated collagen-HA scaffolds into a critical bone defect in male Wistar rats, defects were excised and assessed using microCT. (A) A 6 mm ROI was selected for quantitative analysis of each treatment group; (i) scaffold, (ii) 32-star-PLL-(no gene) scaffold, (iii) 32-star-PLL-pDual scaffold, (iv) 64-star-PLL-(no gene) scaffold and (v) 64-star-PLL-pDual scaffold. (B) Quantitatively, both the 32-star-PLL-pDual scaffold & the 64-star-PLL-pDual scaffold resulted in enhanced new bone volume compared to the scaffold alone group. Outliers have been removed from the analysis using a GRUBBs test.
The field of tissue engineering (TE) has evolved in recent years from the use of biomimetic scaffolds which guide the regenerative process to advanced biotherapeutic-loaded matrices which augment and accelerate tissue repair. These constructs are designed to fill the tissue defect site and provide a physical substrate for tissue growth.
They can also act as a matrix for the controlled delivery of a therapeutic, often to induce autologous host cells to proliferate and differentiate. Within their laboratory, a series of collagen based scaffolds have previously been developed to function as 3D templates for the regeneration of a range of tissues including collagen-chondroitin sulphate (collagen-CS), collagen-hyaluronic acid (HyA), collagen hydroxyapatite (collagen-HA), and collagen-nanohydroxyapatite (collagen-nHA) scaffolds. They have demonstrated the regenerative capacity of a number of these scaffolds in vivo for the healing of both small animal and large animal bone defects.
Commonly, bioactive therapeutics such as small molecule drugs or growth factors are incorporated into these scaffolds to augment their regenerative capacity.This is particularly evident in the field of bone tissue regeneration, a tissue that traditionally represents a significant challenge to efficiently heal in modern orthopedics. Of these growth factors, BMP-2 is considered to be the most potent osteoinductive factor due to its ability to promote in vitro bone repair as well as being effective in the treatment of pre-clinical human fractures.VEGF is commonly used to encourage the direct formation of blood vessels within the scaffold as it is traditionally associated with an angiogenic action. Furthermore, VEGF is known to play a key role in osteogenesis via a direct action on osteoblasts as well as having a pivotal role in fracture repair.
Indeed, it is now established that for the successful recapitulation of bone in vivo, the presentation of multiple growth factors at the defect site is likely to result in enhanced functional tissue regeneration. While many growth factors co-operate during the bone formation process, the co-application of BMP-2 (pro-osteogenic) and VEGF (pro-angiogenic) growth factors has been shown to possess a potent, synergistic effect in mimicking the angiogenic–osteogenic coupling necessary for the formation of vascularized bone.
While the dual delivery of therapeutic proteins to a tissue defect on scaffold based constructs is promising, it remains hindered by the repeated, supraphysiological doses of proteins required which can often result in the formation of ectopic tissue. As a result, there is increasing interest in the design of gene-activated scaffolds, advanced implantable platforms which are capable of delivering gene therapeutics in a controlled and localized manner at the defect site.This promising combination of gene therapy and TE relies on the use of vectors which are capable of transfecting autologous host cells to induce in vivo protein expression. This in vivo expression provides a physiologically relevant protein dose which is localized at the defect site.
A critical aspect to the translation of gene-activated scaffolds is a biocompatible and efficient vector which is capable of efficiently transfecting infiltrating autologous host cells in vivo within the 3D matrix. A number of concerns associated with the use of viral based vectors, such as prolonged and expensive manufacturing costs, the risk of toxicity, immunogenicity and insertional mutagenesis has focused the field on the design of synthetic, bioinspired non-viral vector systems.Since the seminal works on gene activated scaffolds,numerous non-viral gene delivery vectors have been evaluated for TE. Despite the multitude of potential non-viral vector candidates, few have been successfully translated in vivo due to underlying limitations with the vector themselves such as toxicity or poor transfection efficiency.
Star-polypeptides are a broad class of branched polymeric architectures which consist of linear polypeptide arms radiating from a central core. Previously, they have extensively described a novel class of bioinspired star-shaped poly(l-lysine) polypeptides with varying number and length of attached poly(l-lysine) arms referred to as star-PLLs. They have demonstrated that star-PLLs are capable of rapidly self-assembling with plasmid DNA (pDNA) to form a nanomedicine. These nanomedicines can facilitate non-toxic, efficient transfection of mesenchymal stem/stromal cells (MSCs) with subsequent bioactive, therapeutic protein expressionIntracellular delivery of the star-PLL-pDNA complex to MSCs is achieved via a claritin independent internalization process. Furthermore, they have highlighted the capacity of star-PLLs to effectively functionalize a range of collagen based scaffolds in vitro and function as a biocompatible nanomedicine depot for reporter genes in vivo. These star-PLL functionalized scaffolds were capable of facilitating autologous host cell transfection at the early timepoint of just 7 days post implantation.
Building upon their previous work, this study aimed to create for the first time a therapeutically active, cell-free, gene activated scaffold which is specifically tailored for the rapid regeneration of bone tissue using the star-PLL nanomaterials. Two star-PLL compositions were evaluated during this study which encompassed structural variations to the polypropylene imine (PPI) dendrimer core generation (4th generation or 5th generation), the poly(l-lysine) arm number (32 arms or 64 arms) and the number of poly(l-lysine) subunits per arm number (40 subunits or 5 subunits) namely; G4(32)PLL40 (32-star-PLL) & G5(64)PLL5 (64-star-PLL). Initially, they optimized the star-PLL-pDNA gene activated scaffold platform for the osteogenic differentiation of MSCs in vitro by varying the gene cargo delivered and the macromolecular composition of the scaffold used. Following identification of a lead platform for bone tissue repair they assessed the translational potential of these optimized star-PLL-pDNA gene activated scaffolds in vivo. In these studies their ability to accelerate the healing of a critical sized rodent (rat) calvarial bone defect in vivo at an early timepoint of 4-weeks post implantation was evaluated1.
Walsh DP, Raftery RM, Murphy R, Chen G, Heise A, O'Brien FJ, et al.. Gene activated scaffolds incorporating star-shaped polypeptide-pDNA nanomedicines accelerate bone tissue regeneration in vivo. Biomaterials Science 2021. doi:10.1039/d1bm00094b.






Kommentare