Peripheral artery disease is a progressive, devastating disease that leads to critical limb ischemia (CLI). Therapeutic angiogenesis using stem cell therapy has emerged as a promising approach for its treatment; however, adapting cell-based therapy has been limited by poor cell survival and low treatment efficiency. To overcome unmet clinical needs, they developed a fibroblast growth factor 2 (FGF2)-immobilized matrix that enabled control of cell adhesion to the surface and exerted a priming effect on the cell. Human adipose-derived stem cells (hASCs) grown in this matrix formed a functionally enhanced cells spheroid (FECS-Ad) that secreted various angiogenic factors including interleukin-8 (IL-8). They demonstrated that IL-8 was upregulated by the FGF2-mediated priming effect during FECS-Ad formation. Immobilized FGF2 substrate induced stronger IL-8 expression than soluble FGF2 ligands, presumably through the FGFR1/JNK/NF-κB signaling cascade. In IL-8-silenced FECS-Ad, vascular endothelial growth factor (VEGF) expression was decreased and angiogenic potential was reduced. Intramuscular injection of FECS-Ad promoted angiogenesis and muscle regeneration in mouse ischemic tissue, while IL-8 silencing in FECS-Ad inhibited these effects. Taken together, their data demonstrate that IL-8 contributes to therapeutic angiogenesis and suggest that FECS-Ad generated using the MBP-FGF2 matrix might provide a reliable platform for developing therapeutic agents to treat CLI.

The MBP-FGF2 protein was immobilized on polystyrene plates. This biologically modified substrate could trigger an intracellular signaling pathway through binding with the membrane receptor, FGFR1. It also contributed to forming cellular spheroids by adhering to HSPG to control cell-matrix adhesion strength.
Critical limb ischemia (CLI) is the most severe clinical manifestation of peripheral arterial disease (PAD), which is caused by atherosclerotic changes in arteries supplying blood to the lower extremities. Prolonged ischemic conditions frequently lead to disability and amputation of the affected limb, which greatly reduces quality of life. The currently available clinical treatments for this disease are endovascular or surgical revascularization, which are limited by morbidity and mortality complications. Therefore, there is an urgent need for novel therapeutic strategies for patients with CLI. The term “therapeutic angiogenesis” refers to biological intervention to stimulate the creation of new blood vessels in ischemic organs or tissues by administering angiogenic factors. Several factors tightly regulate vascularization and angiogenic progress. Well-characterized angiogenic factors include vascular endothelial growth factor (VEGF), angiopoietins, fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and platelet-derived growth factor (PDGF), although dozens of other proteins contribute to the different stages of blood vessel formation. For example, VEGF plays a role in increasing the number of capillaries, and angiopoietins contribute to blood vessel stability and maturation4. Under normal physiological conditions, most of these secretory factors are tethered to components of the extracellular matrix (ECM), including heparan sulfate proteoglycans (HSPGs) and fibronectin. During tissue injury, mechanical or biological stimuli such as low oxygen concentration (hypoxia) induce the cleavage of ECM by hydrolytic enzymes to release proangiogenic factors toward the damaged tissue. Strategies for therapeutic angiogenesis have been widely investigated for application in diverse human diseases. These include nucleotide or protein delivery and cell transplantation. However, treatments for therapeutic angiogenesis have not yet been approved by the U.S. Food and Drug Administration. Recent advances in stem cell therapy have recognized the therapeutic potential of mesenchymal stem cells (MSCs) for ischemic diseases, such as CLI and myocardial infarction.

A–C Characterization of 3D-As. A Size ranges of FECS-Ad and 3D-A96. n = 33 per group. B Shape distribution of 3D-As. n = 33 per group. C Digital optical, phase contrast, and SEM images of FECS-Ad formed in a 384-well microplate, Scale bar, 200 μm. D–F hASCs were cultured on MBP-FGF2 surface, under hypoxia, or using the hanging drop method for 24 h. Conditioned medium were prepared followed by ELISA. D IL-8 protein expression in FECS-Ad. ****p < 0.0001 (unpaired Student’s t test), n = 6 per group. E Effect of hypoxic condition on IL-8 protein. ns not significant (one-way ANOVA), n = 6 per group. F IL-8 production in 3D spheroids generated by the hanging drop. ****p < 0.0001 (one-way ANOVA), n = 6 per group. G–I hASCs were cultured on MBP-FGF2 surface in a monolayer manner for appropriate time points. Total RNA, protein, and conditioned medium were prepared and analyzed by RT-qPCR and ELISA, respectively. G IL-8 RNA expression on MBP-FGF2 matrix. Values were normalized to GAPDH. ****p < 0.0001 (one-way ANOVA), n = 3 per group. H IL-8 protein expression in hASCs attached to MBP-FGF2 surface. ****p < 0.0001 (one-way ANOVA), n = 3 per group. I IL-8 protein secretion on MBP-FGF2 surface. ****p < 0.0001 (one-way ANOVA), n = 3 per group. All data are presented as mean ± SD.
In particular, human adipose-derived stem cells (hASCs) have gained particular attention since they are readily available, abundantly supplied among MSCs, and also retain immunoprivileged properties and the ability to differentiate multiple cell lineages. They also reportedly promote angiogenesis by secreting various angiogenic factors. Despite the advantages of these cell sources, unsolved limitations regarding their clinical application remain. These mainly include insufficient therapeutic efficacy and poor cell survival rates due to limited well-organized vascular tissue at the transplanted site.

hASCs were cultured on MBP-FGF2 surface, or treated with soluble form of FGF2, or MBP-FGF2 proteins. Total RNAs, proteins, and conditioned medium were prepared and analyzed by RT-qPCR, western blot, and ELISA, respectively. A Effect of various FGF2 ligands on IL-8 RNA. Values were normalized to GAPDH. ns not significant, ***p < 0.001, ****p < 0.0001 (one-way ANOVA), n = 3 per group. B Effect on IL-8 protein. ***p < 0.001, ****p < 0.0001 (one-way ANOVA), n = 3 per group. C Effect on JNK phosphorylation. D Effect on p65 phosphorylation. For western blot analysis, β-actin was used as a loading control. All data are presented as mean ± SD.
Additionally, cells administered into the ischemic region are exposed to hypoxia, causing cell apoptosis. Several methods have been developed to overcome these therapeutic angiogenesis limitations, including: (1) transplanting cells with additional cell-supporting components, (2) genetically modifying cells to enhance functionality and survival, and (3) three-dimensional (3D) cell clustering. However, current methods utilizing transduced cells or cells combined with growth factors are extremely limited as they can potentially show immunogenicity and oncogenicity. On the other hand, 3D cell clusters, especially in scaffold-free platforms, reportedly exert therapeutic effects in ischemic diseases by inducing cell growth and survival without such safety issues. Among several 3D clustering platforms, physically modified surfaces, such as low attachment and patterned surfaces, were utilized for cell spheroid formation as they are relatively easy to control and suitable for the formation of uniform 3D structures without shear stress. They designed a biologically modified surface to realize a functionally enhanced cell spheroid (FECS) for high-quality cell therapeutics, a biologically modified surface plays multiple roles in enhancing cell function through cell membrane receptor signaling and forms cellular spheroids through control of the cell-matrix adhesion strength. They previously developed a novel surface with biological functionality: the maltose-binding protein-fused basic fibroblast growth factor (MBP-FGF2)-coated surface. MBP is a periplasmic receptor for maltose transport, originally found in Gram-negative bacteria. MBP-fusion protein expression systems are commercially available and have been used for purification of recombinant proteins. Since MBP has considerable hydrophobic residues on its surface, the protein enables MBP-fused bioactive molecules to coat hydrophobic polystyrene (PS) plates. They demonstrated that various MBP-fused proteins, including MBP-FGF2, could be coated onto a PS surface as a monolayer.

hASCs were treated with FGFR1 siRNA or PD173074, an FGFR1 inhibitor, and cultured on NTCP or MBP-FGF2 surface for 24 h. Total RNA and conditioned medium were prepared and analyzed by RT-qPCR and ELISA, respectively. A Effect of FGFR1 knockdown on IL-8 RNA. B Effect of FGFR1 knockdown on IL-8 protein. C Effect of PD173074 on IL-8 RNA. D Effect of PD173074 on IL-8 protein. For all data, ****p < 0.0001 (one-way ANOVA), n = 3 per group. Values were normalized to GAPDH for RT-qPCR analysis. All data are presented as mean ± SD.
They previously reported that hASCs could uniformly form 3D spheroids, which they named FECS-Ad, on this surface. FGF2 in the MBP-FGF2-coated surface mediated coupling with the cell surface in a heparan sulfate proteoglycan (HSPG)-dependent manner, which led to reduced cell-to-matrix adhesion. The biophysical balance between cell-to-cell contact and cell-to-surface adhesion force may be involved in the generation of 3D spheroids by inducing aggregation of cells. Cells in 3D culture, including spheroids, secrete various growth factors and cytokines, but the mechanisms by which this occurs remain to be elucidated. FECS-Ad secreted various angiogenic factors, including VEGF, FGF2, HGF, and IL-8 in vitro, and promoted angiogenesis in a mouse ischemic model, the principle of enhanced cell function in FECS-Ad was hypothesized to be due to 3D formation, hypoxia, and FGF2-mediated cell signaling. They previously demonstrated that VEGF expression was regulated by hypoxia induced during 3D clustering, and other studies have reported that HGF was induced by 3D formation-mediated stimuli. It has been reported that IL-8, an emerging regulator of angiogenesis, might be regulated via growth factor signaling, such as the FGF2 signaling pathway. FGF2 is a well-characterized growth factor involved in various cellular responses, including embryonic development, cell growth, and tissue repair.
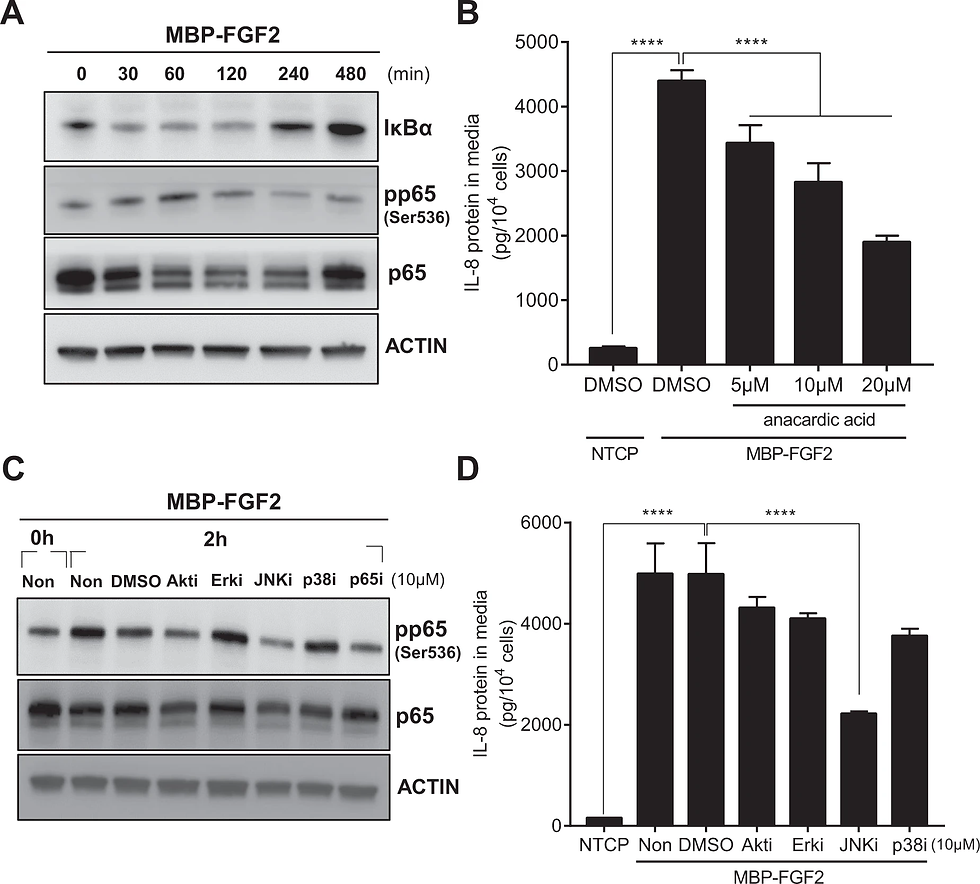
hASCs were cultured on MBP-FGF2 surface in the presence of various inhibitors for downstream molecules. Total proteins and conditioned medium were prepared and analyzed by western blot and ELISA, respectively. A Time-dependent activation of NF-κB signaling pathway of hASC on the MBP-FGF2 surface. B Effect of anacardic acid on IL-8 protein expression. ****p < 0.0001 (one-way ANOVA), n = 4 per group. C Effect of various chemical inhibitors on the MBP-FGF2-mediated increase of phosphorylated p65. D Effect of chemical inhibitors on the MBP-FGF2-mediated regulation of IL-8 expression. ****p < 0.0001 (one-way ANOVA), n = 4 per group. For western blot analysis, β-actin was used as a loading control. All data are presented as mean ± SD.
Interaction of FGF2 with its cellular receptors, primarily FGFR1, activates a variety of signaling pathways such as PI3K, MAPK, and STAT. Further, FGF2 is known to bind to HSPGs, surface molecules that play important roles in cell motility and organization. Binding of FGF2 to HSPGs reportedly boosted the signaling cascade by stabilizing the FGF2-FGFR1 complex. In this study, they investigated the angiogenic potential of FECS-Ad in a murine hindlimb ischemia model, and explored IL-8 function in this process. Their study findings suggested that FECS-Ad and MBP-FGF2 may provide valuable inspiration for developing stem cell therapeutics based on bio-functional materials for treatment of CLI.

A–D hASCs were treated with FGFR1 siRNA or reparixin, an IL-8 receptor inhibitor, and cultured on MBP-FGF2 surface to form FECS-Ad. Conditioned medium from FECS-Ad were prepared and analyzed by ELISA. A Knockdown efficiency of IL-8 protein in FECS-Ad. ns not significant, ****p < 0.0001 (two-way ANOVA), n = 3 per group. Effect of IL-8 knockdown on B VEGF and C HGF protein. ns not significant, ****p < 0.0001 (two-way ANOVA), n = 3 per group. D Effect of reparixin on VEGF protein. ns not significant, ***p < 0.001 (two-way ANOVA), n = 3 per group. E–G GFP-HUVECs plated on matrigel were co-cultured with hASCs, FECS-Ad, or IL-8 silenced FECS-Ad in transwell plate for 16 h. E Effect of hASC co-culture on the tube formation of HUVECs. F Effect on the total tube length of HUVECs. **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA), n = 5 per group. G Effect on the number of branching points of HUVECs. **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA), n = 5 per group. All data are presented as mean ± SD.

One day after HLI induction, mice were i.m. injected with PBS, FECS-Ad, or IL-8-silenced FECS-Ad. Morphometric analysis and blood perfusion was assessed at 7, 14, 21, and 28 days post-surgery. A Representative images of hindlimb morphology and blood perfusion of each group. n = 6 per group. B Blood perfusion ratio (percentage of normal limb) of ischemic limbs quantitated by laser Doppler imaging. Asterisk denotes significant difference compared to PBS group (p < 0.0001) and hash denotes significant difference compared to FECS-Ad (IL-8 KD) group (p < 0.0001) (two-way ANOVA). n = 6 per group. C Physiological status of ischemic limbs assessed at 28 days post-surgery. n = 6 per group. All data are presented as mean ± SEM.

Ischemic thigh and TA muscles isolated from PBS, FECS-Ad, or IL-8-silenced FECS-Ad-injected mice were immunostained with HNA and CD31. Nuclei were stained with DAPI. HNA and CD31 staining of A ischemic thigh and B TA of each group at 1, 7, 14, and 28 days post-surgery. n = 5 per group. Scale bar, 20 μm. C Ratio of HNA-positive cells in ischemic thigh. FECS-Ad group at day 7 was presented as 100%. ns not significant between FECS-Ad and FECS-Ad (IL-8 KD) group, *p < 0.0001 compared to PBS group (two-way ANOVA). n = 5 per group. Percentage of CD31-positive cells in D ischemic thigh and E TA. Normal group was presented as 100%. ns not significant between PBS and FECS-Ad group, *p < 0.0001 compared to PBS group, #p < 0.0001 compared to FECS-Ad (IL-8 KD) group (two-way ANOVA). n = 5 per group. All data are presented as mean ± SEM.

Ischemic thigh and TA muscles from PBS, FECS-Ad, or IL-8-silenced FECS-Ad-treated mice were prepared followed by immunofluorescence. A Representative TAs at appropriate times after ischemic surgery are shown in the photos. n = 4 per group. Scale bar, 2 mm. B Time kinetics of TA weights of each group 1, 7, 14, and 28 days after surgery. ns not significant among PBS, FECS-Ad, and FECS-Ad (IL-8 KD) group, *p < 0.0001 compared to PBS group, #p < 0.0001 compared to FECS-Ad (IL-8 KD) group (two-way ANOVA). n = 4 per group. Laminin staining of C ischemic thigh and D TA of each group at 1, 7, 14, and 28 days post-surgery. Nuclei were stained with DAPI. Scale bar, 20 μm. n = 5 per group. Quantification of muscle fibers with at least one centralized nuclei in E ischemic thigh and F TA. ns not significant among PBS, FECS-Ad, and FECS-Ad (IL-8 KD) group, *p < 0.0001 compared to PBS group, #p < 0.0001 compared to FECS-Ad (IL-8 KD) group (two-way ANOVA). n = 5 per group. All data are presented as mean ± SEM.

Proposed model for IL-8 production in FECS-Ad and its therapeutic potential in ischemic diseases. MBP-FGF2 binds to the FGFR1 of hASCs, activates JNK-NF-κB cascade to produce IL-8. IL-8 secreted from FECS-Ad not only acts on hASCs in an autocrine manner to induce VEGF secretion, but also contributes to the formation of tubular structure of endothelial cells. The red solid line is defined in this study, and the dotted line is already known through reference literature.
Choi, J., Choi, W., Joo, Y. et al. FGF2-primed 3D spheroids producing IL-8 promote therapeutic angiogenesis in murine hindlimb ischemia. npj Regen Med6, 48 (2021). https://doi.org/10.1038/s41536-021-00159-7



Comments